746
Views & Citations10
Likes & Shares
Tissue and organ maintenance is partly accomplished by proliferation and differentiation of adult progenitor/stem cells, division of differentiated cells and/or conversion of cell lineage. Several studies investigated the implication of the adrenal gland under basal and stress conditions. The adrenal has a central role in the body’s reaction to stress; the proper adaptation of the gland function is pivotal in the body’s stress response and regain of homeostasis. Literature provided evidence to the involvement of intra‐adrenal cortical-medullary interactions in this coordination of the body’s response to stress. It is of increasing significance to elucidate the contribution of adrenal progenitor cells, which is regarded crucial to the gland’s response to different physiological and pathological situations especially in stress adaptation. This review aims at summarizing the current data regarding the effectors of the adaptive stress response, highlighting the implication of cortical-medullary cross talk and the role of adrenal stem cells in emphasizing the gland to cope with the increased demands under stress conditions.
Better understanding of these processes might pave the way for scientists to better understand the mechanisms of reprogramming the stress-inducible stem cells, under the influence of stress hormones and other stressors and their relevance to chronic disease. Special attention should be focused on the possible association between the effect of exposure to stressors in early life and the development of chronic diseases in adulthood, as for childhood being the most dynamic period of development.
Keywords: Stress, Adrenal gland, Cortica-medullary cross talk, Stem cells, Adaptive response
INTRODUCTION
Stress is a state of threatened homeostasis developed by the body in response to different stimuli or adverse factors; these stimuli are collectively called stressors [1].
Extrinsic stressors include environmental factors that are potentially hazardous, being capable of inducing alterations in different biological systems. Intrinsic stressors comprise accumulation of waste products and generation of excessive amounts of Reactive Oxygen Species (ROS) that exceed the anti-oxidant capacity of the cells. These molecules, produced as byproducts during the mitochondrial electron transport of aerobic respiration, are involved in a variety of pathologic events [2].
Thus, stress is considered as a state of disharmony, that is eventually compensated by a systemic cascade of physiologic and behavioral responses which aim to counteract the threatened homeostasis; via the so-called the “adaptive stress response” [3]. Stress can take several forms at the cellular level, subsequently affecting the organism as a whole, being depicted in the form of physiological, biochemical and/or behavioral manifestations. Stress affects an organism’s well-being state, provoking energy-consuming processes to counteract the resulting deleterious effects thus, the individuals could possibly become immune-compromised, with subsequent vulnerability to pathogens. Current drug therapies for stress management are still controversial regarding poor efficacy, withdrawal symptoms and side effects [4]. This “adaptive stress response” may be accompanied by upregulating the concentration of different biochemical compounds; namely hormones particularly cortisol and epinephrine, as well as Reactive Oxygen Species (ROS) and inflammatory cytokines, within the cells or body fluids. This could be reflected clinically as an elevation in relevant biomarkers’ levels, specifically used for diagnosis, prognosis and treatment [5].
Physical adaptation to stress encompasses reactive redirection of energy and body resources, through increases in the cardiovascular tone, pulse rate, respiratory rate, core body temperature and metabolic rate through gluconeogenesis and lipolysis; all acting concomitantly to promote this redirection of vital substrates, while temporarily suppressing energy-consuming functions (e.g. digestion, reproduction, growth and immunity). Thus, oxygen and nutrients are primarily shunted to the CNS and to stressed body site(s) where they are needed the most [6]. Classical stress markers comprise of endocrinal hormonal changes, principally affecting cortisol and epinephrine levels [7].
The adrenal gland is a master regulator of the human body during response to stress. It would be of great significance to address how stress stimulates stem cells in the adrenal gland. In this review, the comprehensive information regarding the neuro-endocrine effectors of the adaptive stress response was summarized, with reference to the human stress response pathways. In addition to highlighting stem cells found in the adrenal gland and the effect of stress on adrenal stem cells, depicting how the coordinated action of stress-inducible stem cells ensures tissue remodeling, cellular and functional adaptation to stress.
EFFECTORS OF THE STRESS RESPONSE: “THE ADAPTIVE STRESS SYSTEM”
The “stress system” is composed of complex neuroendocrine, cellular and molecular components which interact to mediate the stress response; being located in both the central nervous system (CNS) and the periphery. The adaptive response of each individual to stress is governed by a variety of genetic, environmental and developmental factors. Pathological disease states might result if the individual does not respond effectively, through the appropriate stress system; either in the form of inadequate, excessive and/or prolonged reactions [8].
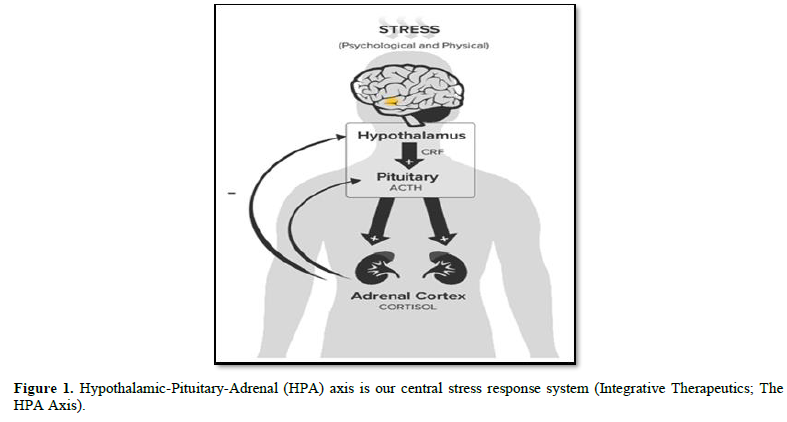
Specific areas of the brain have critical roles in regulating the stress response. The central components of the stress system are located mainly in the hypothalamus. The peripheral limbs of the hypothalamic-pituitary-adrenal (HPA) axis, together with the efferent sympathetic/adreno medullary system, constitute the peripheral components of this interconnected system. The hypothalamic-pituitary-adrenal (HPA)-axis (Figure 1) is the most important neuro-endocrine stress response system of our body which is of critical importance for survival [9].
Endocrine and neural responses to stress involve activation of both the HPA axis and the sympatho-adrenal system. It has become evident that neuro-endocrine systems interact with each other at the level of adrenal gland, and that this interaction is essentially involved in the regulation of adrenal gland function during normal and stress conditions. Proper control of the stress response and the restoration of homeostasis, at proper timing, is of critical importance. Disturbances in HPA axis activity, whether inappropriate or prolonged activation is associated with adverse metabolic and cognitive changes [10].
HUMAN STRESS RESPONSE PATHWAYS
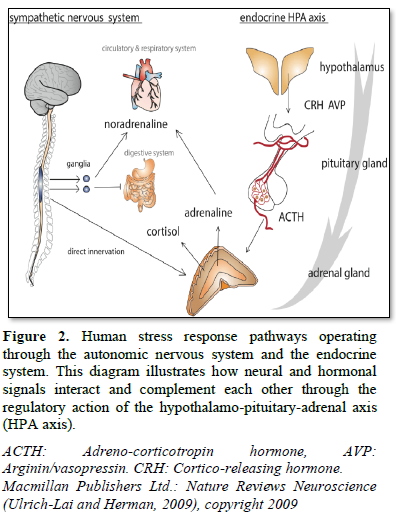
During an acute stress response, a broad category of external and internal stimuli, initiate a cascade of hormonal processes. This cascade starts instantly following stress, in the paraventricular nucleus (PVN) of the hypothalamus, with subsequent release of corticotrophin-releasing hormone (CRH) and arginine-vasopressin (AVP) at the median eminence [11].
CRH in turn is both a central activator of the HPA axis, as well as the sympatho-adrenal system. AVP acts in synergy with CRH to regulate the anterior pituitary ACTH secretion into the blood. ACTH then travels via the systemic circulation to the adrenal cortex, where it triggers the synthesis and release of glucocorticoids (GC) (cortisol in human, corticosterone in rodents). These hormones act widely throughout the body and brain to mobilize energy resources in order to combat stress and regain homeostasis [12]. In addition, GC inhibits unnecessary functions in the early phase of stress response, including growth, digestion, reproduction and immunity [11].
Within seconds, catecholamines (CAs); epinephrine and norepinephrine are produced in the sympathetic nervous system of the adrenal medulla, triggered by splanchnic nerve stimulation. Mutually acting, splanchnic nerve stimulation of the adrenal medulla during an acute stress response will also provoke the release of adrenal glucocorticoids and mineralocorticoids, mediated in a paracrine manner by the released catecholamines. GC is essential to support the action of adrenal medullary catecholamines [13].
REGULATORY CONTROL OF HPA AXIS
In addition to the role of this highly-organized orchestrated cellular and neuronal networks in stimulating and maintaining a proper adrenal stress response, a strict circadian and ultradian control is endowed in regulation of secretion of stress hormones; both centrally and peripherally. The entire endocrine stress system is integrated within a highly complex model of positive and negative feedback regulations, which mature postnatally to become fully functional only after puberty [14].
To avoid overstimulation of the HPA axis, glucocorticoids exert an inhibitory feedback at all stages of the axis; playing a crucial role in the regulation of the basal HPA axis activity and in the precise termination of the stress response. GC performs this inhibitory effect at multiple levels, including extra-hypothalamic regulatory centers, the hypothalamus and the pituitary. Due to the high brain penetrating ability of GC, they act on regions such as hippocampus and prefrontal cortex (tonic inhibition) or amygdala (tonic stimulation). Doing so, the inhibitory glucocorticoid feedback on the ACTH secretory response limits the duration of the total tissue exposure to glucocorticoids, thus minimizing the catabolic, anti-reproductive and immunosuppressive effects of these hormones [15].
However, chronic stress leads to a sustained activation of HPA axis and could induce stress-related disorders such as Alzehimer’s disease [16]. Homeostasis maintenance is compromised, leading to insulin resistance, dyslipidemia, atherosclerosis and massive peripheral inflammation [17].
ADRENAL GLAND MICROENVIRONMENT
Within the adrenal gland, two embryonically distinct endocrine tissues coexist: mesodermally derived, steroid-producing cortex and ectodermal neural crest-derived, catecholamine-producing medulla. The adrenal cortex originates from cells of the celomic epithelium, which at an early stage of development, condense between the urogenital ridge and the dorsal mesentery and form the adrenogonadal primordium. Later during organogenesis, cells of the neural crest migrate into the fetal adrenal, where they become precursors of the adrenal medulla [18].
ADRENAL GLAND MORPHOLOGY
Cellular components of the two regions of the adrenal gland mediate homeostasis, through the release of distinct hormones. The adrenal cortex is divided into three different zones; the outemost zone immediately under the capsule
zona glomerulosa, responsible for secretion of mineral corticosteroids, the glucocorticoid-producing zona fasciculata and the innermost androgen-producing zona reticularis, which surrounds the medulla. The mammalian adrenal cortex predominantly produces steroid hormones, of which glucocorticoids constitute a major group [19].
The catecholamines (CAs); epinephrine and norepinephrine are the major secretory products of the adrenal medulla. The main cell type of the adrenal medulla is the chromaffin cell (or pheochromocytes), named as such owing to the affinity of catecholamines for chromium salts [20]. In addition to chromaffin cells, the adrenal medulla also contains ganglion and glia sustentacular cells. Chromaffin cells, arranged as clusters, store catecholamines in secretory vesicles. At the periphery of those clusters, sustentacular cells are located, while ganglion cells are found individually or in clusters interfused among the chromaffin cells or near nerve fibers. In the normal adrenal gland, steroid and catecholamine secretion is regulated by a complex network of autocrine and paracrine interactions [13].
The adrenocortical tissue is richly innervated by sympathetic fibers originating from extra-adrenal neurons. Other nerve fibers originate from cells in the adrenal medulla and innervate the inner zone of the cortex. The adrenal medulla is highly innervated by preganglionic sympathetic fibers. Following activation of the autonomic nervous system, splanchnic nerve stimulation leads to secretion of the major secretory products of the chromaffin cells; the catecholamines epinephrine and norepinephrine, which are responsible for the execution of the fight-or-flight response of the sympathetic nervous system. Such response involves an increase in blood pressure (resulting in vasoconstriction and increased blood flow to muscles and brain), an increase in the heart rate and contractility, relaxation of smooth muscles in the airways and glycaemia [21].
ADRENAL CORTEX AND MEDULLA CROSSTALK
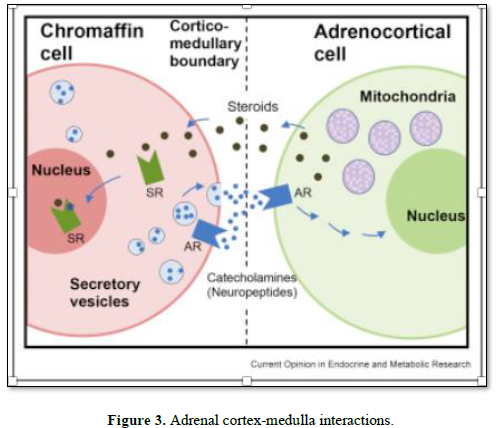
Ample evidence exists suggesting a bidirectional interaction between adrenal cortex and the medulla. Despite a classical view on the adrenal gland anatomy, demonstrating a clear separation of the cortex and medulla, distinct origin and hormone production profiles, it has been well documented that these two endocrine tissues are both morphologically and functionally interwoven and show multiple contact zones without separation by connective tissue or interstitial membranes. In addition, cortical cells are diffusely present in the adrenal medulla and, conversely, chromaffin cells are intermixed with cortical cells within all three zones of the adrenal cortex [22-23]. Within these two environmental niches, interplay between various cells takes place including adrenocortical and chromaffin cells, neuronal cells, immune cells, endothelial cells and glia cells. This cortical-chromaffin crosstalk remains important for physiological regulation of adrenal hormone biosynthesis in adult life and also is relevant for the pathogenesis of various adrenal gland disorders [22].
This close localization, in turn, enables direct cell-cell and paracrine interactions between adrenocortical and chromaffin cells involving their secretory products. Early studies [23] demonstrated that the biosynthesis of adrenomedullary epinephrine is regulated by adrenocortical glucocorticoids. Adrenal cortex-derived glucocorticoids (GCs) were found to enhance synthesis of CAs both in vitro and in vivo. GCs were found to execute these effects by up-regulating expression of phenylethanolamine N-methyltransferase (PNMT), whose gene encodes for an enzyme that catalyzes the synthesis of epinephrine from norepinephrine [24]. Correspondingly, an intact function of adrenal medulla is essential to preserve adrenocortical function. It has been shown that coculture of bovine adrenocortical cells with chromaffin cells stimulated a 10-fold increase in basal GC secretion. Furthermore, CAs were found to regulate the release of various steroids including cortisol, aldosterone and androstenedione, via upregulation of steroidogenic acute regulatory protein [25].
Adrenal medulla-to-cortex regulation is now also accepted to occur via paracrine control achieved by the adrenomedullary chromaffin cells [26]. Chromaffin cells produce various neuropeptides including chromogranins, enkephalins, substance P and neuropeptide Y. These substances are known to interact with various cell types within adrenal gland itself, thereby influencing its function during stress conditions and disease. The majority of these peptides influence the adrenocortical function by enhancing steroidogenesis; however, some peptides such as adrenomedullin, neuropeptide Y and dopamine exert an inhibitory function [27].
Regarding CRH as the main regulator of the HPA axis and steroidogenesis, there exists various CRH and ACTH-independent factors, that are capable of activating the release of adrenal stress steroids, including neuropeptides, cytokines, the microbiota-gut-brain axis and even bacterial and viral pathogens. Likewise, cells of the immune system are in close contact with steroidogenic cells [28]. Moreover, catecholamine and neuropeptide biosynthesis in chromaffin cells is modulated by various vasoactive or proteolytic enzymes, cytokines such as interleukin 1 and 6 from immune cells, as well as by direct cell-cell contacts and activation of toll-like receptors (TLRs) [29].
ADRENAL CORTEX AND MEDULLA INTERACTION DURING STRESS
Adrenal gland, as part of both the HPA axis and the sympatho-adrenomedullary system, is an essential regulator of the acute stress response. The importance of synchronization of the response from the adrenal cortex and the medulla has been studied in animal models. Deleterious consequences on adrenal medullary tissue were reported in case of absence or impairment of the adrenal cortex, including lack of PNMT and secretogranin II in glucocorticoid receptor-deficient mice [30].
Thus, it is not surprising that, patients with disorders of the adrenal cortex such as Addison’s disease or congenital adrenal hyperplasia, display a dysfunction of the adrenal medulla, resulting in an impaired stress response. Therefore, it could be assumed that there might be a joint subset of progenitors in the adrenal medulla and cortex that, in a synergistic manner, share cellular pathways for coordinating regeneration and adaptation to stress [13].
Likewise, cortical-medullary interactions are observed in patients suffering from pheochromocytomas or medullary hyperplasia associated with adrenal adenoma. This leads to catecholamine overproduction in combination with hyperaldosteronism or Cushing’s syndrome [31].
Isolated cases of patients with ectopic ACTH-secreting pheochromocytomas have been documented. Clinical studies reported that pheochromocytoma and paraganglioma patients have increased circulating concentrations of 11-desoxycortisol, 11-desoxycorticosterone, corticosterone and aldosterone, compared with primary hypertensive patients and normotensive controls [32].
ADULT STEM CELLS
Adult progenitor or stem cells are found in variety of organs where they contribute to the renewal of organ‐specific cells. A proper balance between the proliferation and differentiation of such stem/progenitor cells is crucial, as dysregulation of this mechanism might result in organ failure [33].
Stem cells typically reside in protected locations within stem cell niches in different tissues and organs of the body, where they contribute to the renewal of organ-specific cells. Stem cell niches are specific anatomic locations that determine the ability of stem cells to self-renew, participate in tissue generation, maintenance and repair. The niche constitutes a basic unit of tissue physiology, integrating signals that mediate the balanced response of stem cells to the needs of organisms. For certain stem cell types, the niche has been found to be a hypoxic environment, which is expected to reduce oxidative stress in the stem cells [34].
Progenitor and stem cell populations are required for the successful homeostasis and adaptation of most tissues. Mammalian stem cells have several features in common that can be interpreted as “anti-stress protective” mechanisms. They often exhibit upregulated stress response and repair pathways, in the form of increased chaperone expression [35] and increased transporter expression in adult stem cells that may facilitate removal of toxins [36].
Extrinsic stress to the stem cells may be in the form of changes in circulating hormones’ level, as well as environmental stresses that cause tissue and cellular damage. Inflammation, in response to tissue damage, signals stem cell activation and repair in several tissues. Chronic inflammation, such as that associated with aging, is implicated in cancer progression and the maintenance of cancer stem cells. Cancer stem cells are hypothesized to support the growth of specific tumors analogous to the way adult stem cells support normal tissues.
It is assumed that exposure to stress early in life has the capacity to control stem cell fate and lineage commitment, this can have major implications in responding to, as well as managing disease during adulthood [37]. External and internal stressors from the first day of life influence the process of cell differentiation of stem and progenitor cells in the HPA axis to form the fully functional endocrine stress system. Chronic stress in experimental studies, have been shown to stimulate presynaptic and postsynaptic modifications in the paraventricular nucleus of the hypothalamus that are in accordance with increased HPA axis drive. A network of growth factors and transcription factors direct the hypothalamic, pituitary and adrenal progenitor cells to form the mature HPA axis [38].
ROLE OF PROGENITOR/STEM CELLS POPULATIONS IN THE ADRENAL GLAND UNDER STRESS CONDITIONS
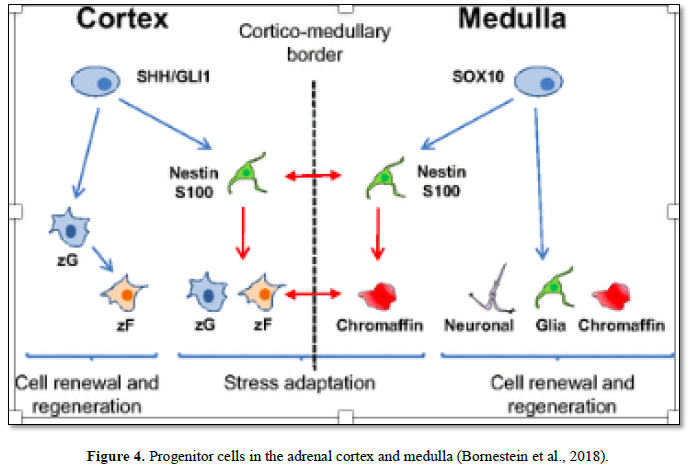
During adrenal organogenesis, close interactions between its two components, medulla and cortex, are necessary for differentiation, morphogenesis, and survival of the adrenal gland. A high degree of plasticity is critical to sustain homeostasis under different physiological demands. The adrenal gland shows constant replacement of aging cells by newly differentiated cells. The continued proliferative capacity of the adult adrenal gland to maintain adrenal volume and function throughout life is consistent with the presence of a stem-like population of cells. Maintenance of the adrenal is partly achieved by division and differentiation of adult progenitors and stem cells located in the adrenal cortex and medulla [39] (Figure 4).
Several studies indicated that the adrenal capsule is more than a static support structure, but rather contains a dynamic population of adrenocortical stem/progenitor cells that serve as precursor cells for the cellular components of the definitive cortex. It is assumed that the adrenal capsule serves as a stem cell niche/residence for adult adrenocortical stem/progenitor cells that reside within and/or underneath the capsule. As such, the role of this capsule in the transition from a fetal to adult cortex and ultimately in the homeostatic maintenance of the adult gland is of great significance [40].
One of the first reports regarding adrenal regeneration in rats applied a methodology of unilateral enucleation of the adrenal medulla of rats, while sparing the capsule and underlying subcapsular cells intact. This was followed by restoration of the adrenal cortex 6 weeks later. This observation suggested the presence of an adrenocortical stem /progenitor cell population at the periphery of the adrenal gland that gives rise to all the steroidogenic cell types of the cortex [41].
Thereafter, several studies have demonstrated that distinct populations of adrenocortical progenitors are located in the subcapsular region of the adrenal cortex, possibly in the Zona glomerulosa, where they contribute to daily cell renewal and regeneration, initially differentiating into Zona glomerulosa cells and then Trans-differentiate into Zona fasciculata cells. Studies have characterized these cells as sonic hedgehog SHH+ cells, representing a population of undifferentiated cells expressing progenitor characteristics [42-43].
Furthermore, a distinct subpopulation of cells were characterized in the adrenal cortex and/or subcapsular region of the adrenal, expressing progenitor-cell markers such as Nestin (acronym for neuroectodermal stem cell marker) is a type IV intermediate filament protein, expressed mostly in nerve cells where it is involved in the radial growth of the axon. Such adrenocortical Nestin-expressing cells were found to be sensitive to stimulation with ACTH, suggesting Nestin as a progenitor marker [44].
Regarding the adreno-medullary stem cells, in vitro studies showed that cells with progenitor profiles could be cultured from bovine [45] and human [46] adrenal medullary extracts; these cells could also generate spheres expressing Nestin. It was proved that these medullary progenitors exhibit multipotential nature, being able to differentiate into the three major lineages of the adrenal medulla; glia, neurons and chromaffin cells, assuming their significant role during stress, being attributed to their differentiating capacity into chromaffin cells [47].
Scientific data provided by proved that Nestin+ adrenocortical progenitors are interconnected and physically interact with Nestin+ medullary stress-dependent progenitors, denoting active cellular and functional interaction between these progenitors within the gland. Although Nestin+ cells in the cortex and medulla form a network of interconnected cells, they belong to two different populations, as their differentiation potentials are different [38].
Under basal conditions, these subcapsular and/or cortical progenitors very slowly migrate centripetally through the different zones of the adrenal cortex to the cortico-medullary boundary. A continual centripetal displacement of cells from the outer periphery of the cortex to the cortical-medullary boundary indicates the peripheral generation of new cortical cells throughout life [33].
However, under stress, the adrenocortical progenitors (which are interconnected with adrenomedullary stress-dependent progenitors), are activated and they mobilize faster from the adrenal cortex. Interestingly, immobilization stress promotes the differentiation of these Nestin-positive progenitor cells into chromaffin cells and has also been shown to alter the expression of catecholamine-producing enzymes and the release of catecholamines. Such findings demonstrate the coordinated action of stress-inducible stem cells to ensure tissue remodeling and cellular and functional adaptation to stress [48]. Impairment of such cortico-medullary communication may lead to adrenal disorders as well as inflammatory, mental, metabolic, neurodegenerative and cardiovascular diseases [49].
CONCLUSION
Stem/progenitor cells of the adrenal gland are localized in the subcapsular area, adrenal cortex and medulla, being involved in the normal turnover process of the aging cells, in addition to playing an important role during the stress response. Nestin+ cortical progenitors are recruited centripetally from the stem cell pool, towards the cortico-medullary junction, where they differentiate into chromaffin cells, to cope with increased demand. Such highly-organized harmony of the coordinated action of stress-inducible stem cells ensures tissue remodeling, cellular and functional adaptation to stress.
We are still in need for more advanced researches to elucidate the exact mechanisms influencing the interactions between stressors and stem/progenitor cells and to explore the mechanisms by which stressors can induce reprogramming of progenitor cells. This in turn may have direct implications for multiple new lines of evidence regarding disease pathogenesis, particularly mechanisms by which early abnormalities in stress hormone regulation may lead to common diseases in later life. The possible implication of stress on immunity and overall mental health should be explored; while trying to elucidate the exact mechanism by which stress contributes to stimulation of stem cells.
ACKNOWLEDGMENT AND DISCLAIMER
The author declares no conflict of interests.
1. Kumar S, Perumal S, Rajagopalan V (2014) Therapeutic effect of bone marrow mesenchymal stem cells on cold stress-induced changes in the hippocampus of rats. Neural Regen Res 9: 1740-1744.
2. Tower J (2012) Stress and stem cells. Wiley Interdiscip Rev Dev Biol 1: 789-802.
3. Tsigos C, Kyrou I, Eva Kassi E, Chrousos GP (2016) Stress, endocrine physiology and pathophysiology. Endotext Comprehensive Free Online Endocrinology Book. Available online at: www.endotext.org
4. Takahashi S, Nakasatomi M, Takei Y, Ikeuchi H, Sakairi T, et al. (2018) Identification of urinary activin A as a novel biomarker reflecting the severity of acute kidney injury. Sci Rep 8: 5176.
5. Dhama K, Latheef SK, Dadar M, Samad HA, Munjal A, et al. (2019) Biomarkers in stress related diseases/disorders: Diagnostic, prognostic and therapeutic values. Front Mol Biosci 6: 91.
6. Carboni L (2013) Peripheral biomarkers in animal models of major depressive disorder. Dis Markers 35:33-41.
7. Ewert A, Chang Y (2018) Levels of nature and stress response. Behav Sci 8: 49.
8. Chrousos GP (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374-381.
9. Malenka, Robert C, Nestler, Eric J, Hyman, Steven E (2009) Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. New York: McGraw-Hill Medical. pp: 246, 248-259.
10. Goldstein DS (2010) Adrenal responses to stress. Cell Mol Neurobiol 30: 1433-1440.
11. Canet G, Hernandez C, Zussy C, Chevallier N, Desrumaux C, et al. (2019) Is AD a stress-related disorder? Focus on the HPA axis and its promising therapeutic targets. Front Aging Neurosci 11: 269.
12. Herman JP, Tasker JG (2016) Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne) 7: 137.
13. Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, et al. (2016) Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 101:364-389.
14. Fitzsimons CP, Herbert J, Schouten M, Meijer OC, Lucassen PJ, et al. (2016) Circadian and ultradian glucocorticoid rhythmicity: Implications for the effects of glucocorticoids on neural stem cells and adult hippocampal neurogenesis. Front Neuroendocrinol 41: 44-58.
15. De Kloet ER (2000) Stress in the brain. Eur J Pharmacol 405: 187-198.
16. Canet G, Chevallier N, Zussy C, Desrumaux C and Givalois L (2018) Central role of glucocorticoid receptors in Alzheimer’s disease and depression. Front Neurosci 12: 739.
17. Maslov LN, Naryzhnaya NV, Boshchenko AA, Popov SV, Ivanov VV, et al. (2019) is oxidative stress of adipocytes a cause or a consequence of the metabolic syndrome? J Clin Transl Endocrinol 15: 1-5.
18. Lerario AM, Finco I, LaPensee C, Hammer GD (2017) Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Front Endocrinol (Lausanne) 8: 52.
19. Morohashi K, Zubair M (2011). The fetal and adult adrenal cortex. Mol Cell Endocrinol 336: 193-197.
20. Bornstein SR, Ehrhart-Bornstein M, Androutsellis-Theotokis A, Eisenhofer G, Vukicevic V, et al. (2012) Chromaffin cells: The peripheral brain. Mol Psychiatry 17: 354-358.
21. Mariniello K, Ruiz-Babot G, McGaugh EC, Nicholson JG, Gualtieri A (2019) Stem cells, self-renewal and lineage commitment in the endocrine system. Front Endocrinol.
22. Bornstein SR, Berger I, Scriba L, Santambrogio A, Steenblock C (2019) Adrenal cortex-medulla interactions in adaptation to stress and disease. Curr Opinion Endocr Metab Res 8: 9-14.
23. Wurtman RJ, Axelrod J (1966) Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem 241: 2301-2305.
24. Sharara-Chami RI, Joachim M, Pacak K, Majzoub JA (2010) Glucocorticoid treatment-Effect on adrenal medullary catecholamine production. Shock 33: 213-217.
25. Haidan A, Bornstein SR, Liu Z, Walsh LP, Stocco DM (2000) Expression of adrenocortical steroidogenic acute regulatory (StAR) protein is influenced by chromaffin cells. Mol Cell Endocrinol 165: 25-32.
26. Ehrhart-Bornstein M, Bornstein SR (2008) Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci 1148: 112-117.
27. Eiden LE, Jiang SZ (2018) what’s new in endocrinology: The chromaffin cell. Front Endocrinol (Lausanne) 9: 711.
28. Kanczkowski W, Sue M, Bornstein SR (2017) The adrenal gland microenvironment in health, disease and during regeneration. Hormones (Athens) 16: 251-265.
29. Bornstein SR, Ziegler CG, Krug AW, Kanczkowski W, Rettori V, et al. (2006) The role of toll-like receptors in the immune-adrenal crosstalk. Ann N Y Acad Sci 1088: 307-18.
30. Haase M, Willenberg HS, Bornstein SR (2011) Update on the corticomedullary interaction in the adrenal gland. Endocr Dev 20: 28-37.
31. Petramala L, Concistre A, Olmati F, Saraceno V, Iannucci G, et al. (2017) Silent adrenal pheochromocytoma coexistent with corticomedullary hyperplasia: A case incidentally discovered. Eur J Case Rep Intern Med 4: 000714.
32. Langton K, Gruber M, Masjkur J, Steenblock C, Peitzsch M, et al. (2018) Hypertensive crisis in pregnancy due to a metamorphosing pheochromocytoma with post-delivery Cushing’s syndrome. Gynecol Endocrinol 34: 20-24.
33. Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, et al. (2009) In search of adrenocortical stem and progenitor cells. Endocrinol Rev 30: 241-263.
34. Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7: 380-390.
35. Steidl U, Bork S, Schaub S, Selbach O, Seres J, et al. (2004) Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood 104: 81-88.
36. Vinogradova TV, Chernov IP, Monastyrskaya GS, Kondratyeva LG, Sverdlov ED (2015) Cancer stem cells: Plasticity works against therapy. Acta Naturae 7:46-55.
37. Andoniadou CL (2016) Pituitary stem cells during normal physiology and disease. Stem cells in Neuroendocrinology. Springer International Publishing AG: Cham (CH), pp: 103-111.
38. Cox B, Roose H, Vennekens A, Vankelecom H (2017) Pituitary stem cell regulation: Who is pulling the strings? J Endocrinol 234: R135-R158.
39. Steenblock C, Maria F de Celis R, Luis F Silva D, Pawolski V, Brennand A, et al. (2018) Isolation and characterization of adrenocortical progenitors involved in the adaptation to stress. Proc Natl Acad Sci U S A 115: 12997-13002.
40. Wood MA, Hammer GD (2011). Adrenocortical stem and progenitor cells: Unifying model of two proposed origins. Mol Cell Endocrinol 336: 206-212.
41. Ingle D, Higgins G (1938) Auto transplantation and regeneration of the adrenal gland. Endocrinology 22: 458-464.
42. Guasti L, Paul A, Laufer E, King P (2011) Localization of sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol Cell Endocrinol 336: 117-122.
43. Finco I, Lerario AM, Hammer GD (2018) Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology 159: 579-596.
44. Balyura M, Gelfgat E, Steenblock C, Androutsellis-Theotokis A, Ruiz-Babot G, et al. (2018) Expression of progenitor markers is associated with the functionality of a bioartificial adrenal cortex. PLOS One 13:
e0194643.
45. Chung KF, Sicard F, Vukicevic V, Hermann A, Storch A, et al. (2009) Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem Cells 27: 2602-2613.
46. Santana MM, Chung KF, Vukicevic V, Rosmaninho-Salgado J, Kanczkowski W, et al. (2012) Isolation, characterization and
differentiation of progenitor cells from human adult adrenal medulla. Stem Cells Transl Med 1: 783-791.
47. Rizzoti K, Akiyama H, Lovell-Badge R (2013) Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 13: 419-432.
48. de Celis RMF, Garcia-Martin R, Wittig D, Valencia GD, Enikolopov G, et al. (2015) Multipotent glialike stem cells mediate stress adaptation. Stem Cells 33: 2037-2051.
49. de Celis MFR, Bornstein SR, Androutsellis-Theotokis A, Andoniadou C, Licinio J (2016) The effects of stress on brain and adrenal stem cells.
Mol Psychiatry 21: 590-593.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Spine Diseases